Website goal
The aim of this website is to provide a central location for background and recent findings on thiamine deficiency and will be continually updated. Please contact Katie Edwards ![]() if you have suggestions.
if you have suggestions.
Thiamine (vitamin B1, thiamin) is essential to the health of all living organisms. In its diphosphate form, thiamine serves as a cofactor for enzymes involved in carbohydrate metabolism as well as the necessary breakdown of certain amino acids and fatty acids. These enzymes help to generate energy in the form of ATP, intermediates needed for the generation of nucleic acids and amino acids, and prevent the build-up of other amino acids that can cause deleterious effects.
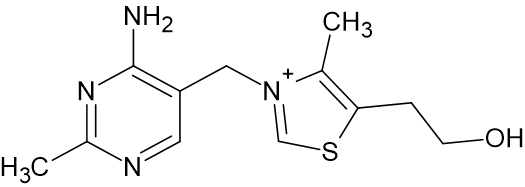
Thiamine is highly water soluble and thus it is not retained in the body. Only ~30 mg is present in the bodies of adults and, since people and other mammals cannot synthesize thiamine, it must be continually obtained from dietary sources. Thiamine is produced in the foliage of many plants important to the health of people, domesticated livestock, and wildlife. These sources include vegetables, grasses, and trees, with elevated content in whole grains and legumes. Certain meats also provide a good source of thiamine, most notably pork livers. The recommended daily allowance (RDA) varies based on age, gender, and state of pregnancy, but generally, between 1.1 and 1.2 mg per day is needed for adults.1
Deficiencies in thiamine result in neurological, muscular, and cardiac symptoms than span organisms ranging from fish to people. These deficiencies result from dietary insufficiences; conditions which case an inability to absorb available thiamine; anti-thiamine factors including enzymes which break down thiamine (thiaminases) or chemical compounds such as sulfites or tannins that either break down or complex with thiamine causing its decreased bioavailability. Conditions associated with thiamine deficiency include diseases such as beriberi and the Wernicke-Korsakoff syndrome in the clinical realm; polioencephalomalacia and Chastek's paralysis in the veterinary realm; and the M74 and Cayuga syndromes in aquatic species. As it is considered a minimum requirement for a basic healthcare system, thiamine is on the World Health Organization's (WHO) core list of essential medicines for both adults and children.2
As early as 2697 B.C., the symptoms of thiamine deficiency associated with beriberi disease were documented in China.3,4 Beriberi affects the heart, circulatory, and nervous systems and symptoms include tremors, muscle weakness, paralysis, and death. In 1878, the emperor of Japan fell ill with beriberi (or kakke in Japanese) and established the Beriberi hospital in an effort to identify the cause of this disease.5 While some association with diets lacking meat was noted, a significant emphasis was placed on the environment of those afflicted. Factors postulated to contribute to beriberi included poor hygiene, poor sanitation, overcrowding, and elevated ambient temperatures. From 1878 to 1882, a large proportion of Japanese naval personel (between 25% and 40%) were afflicted by beriberi.6 The association of beriberi with a protein-deficient diet was solidified by Kanehiro Takaki, a Japanese surgeon, when the symptoms were ameliorated by supplementing the primarily white rice diet of the Japanese navy with meat, fish, condensed milk, and vegetables.4 By altering the rice diet to include barley as a standard, the incidence of beriberi in the navy dropped to 0.04% by 1886.5
Despite this strong correlation, a competing hypothesis was a bacterial infection which sidelined the dietary association noted by Takaki. The theory that calories from protein, fat, and carbohydrates were equivalent and that excesses in one could make up for deficiencies in another (the "law of isodynamic equivalence") also was developed which detracted from the findings of Takaki. Further, the shunning of traditional medicine, of which the dietary supplementation by Takaki was classified, in the military led to the maintenance of the rice diet in the Japanese army.
The dietary impact of beriberi became more pronounced during the Sino-Japanese war (1894-1895) with the continued barley supplementation in the Japanese navy, but not the army. More than 30,000 cases of beriberi in the army occurred, with nearly 2,000 deaths, but no cases in the navy.6 Further evidence for the dietary link was observed in the army in Taiwan with a marked reduction in cases when barley was instituted briefly.
Separately and around the same time, outbreaks of beriberi were prevalent in the East Indies colonies of the Dutch. These symptoms were originally attributed to toxins produced by microbial contamination. Shortly thereafter in 1897, polyneuritis, another condition yielding similar symptoms in poultry, was found by the Dutch physician Christiaan Eijkman to develop when chickens were fed polished white rice, but symptoms were ameliorated when fed the components removed during polishing. He concluded that the hulls of the rice contained an "anti-beriberi factor" which rendered the rice non-toxic. Followed up by studies on human prisoners by Adolphe Vorderman, the further association of polished rice diets with beriberi was made. The loss of a nutritional component through removal of the hulls was later made in 1901 by Gerrit Grijns, Eijkman's assistant.6
As the dietary link remained contentious in Japan, the inclusion of barley in the diets of servicemen in the Japanese army remained an unadopted practice. During the Russo-Japanese war (1904-1905), more than 250,000 cases of beriberi with 27,000 deaths occurred.5 Under limited distribution, improvement was again noted with the dietary inclusion of barley, but there remained disagreement regarding the cause of the improvement. An association was gradually made between rice and beriberi, but one theory was that the high carbohydrate content facilitated growth of the microorganism responsible for the disease, still largely attributed to a contagious agent. Despite mounting evidence to the dietary and not microorganismal link, the disagreement over causation continued in Japan for years.
In 1906, the English biochemist Frederick Hopkins identified certain "accessory factors", later realized to be vitamins, beyond the carbohydrates, fats, and proteins in foods which were necessary for maintenance of health. The "anti-beriberi factor" present in whole rice, coined "vitamine" by Polish biochemist Casimir Funk in 1912 who originally attempted its isolation, was later isolated in purified form from rice bran in 1926 by the Dutch biochemists Barend Jansen and Willem Donath.6 Its structure was elucidated and chemical synthesis developed by the American chemist Roger Williams in 1936. Eijkman and Hopkins were awarded the Nobel Prize in 1929 for their "anti-beriberi factor" and identification of "accessory factors", later realized to be vitamins, in foods, respectively.7
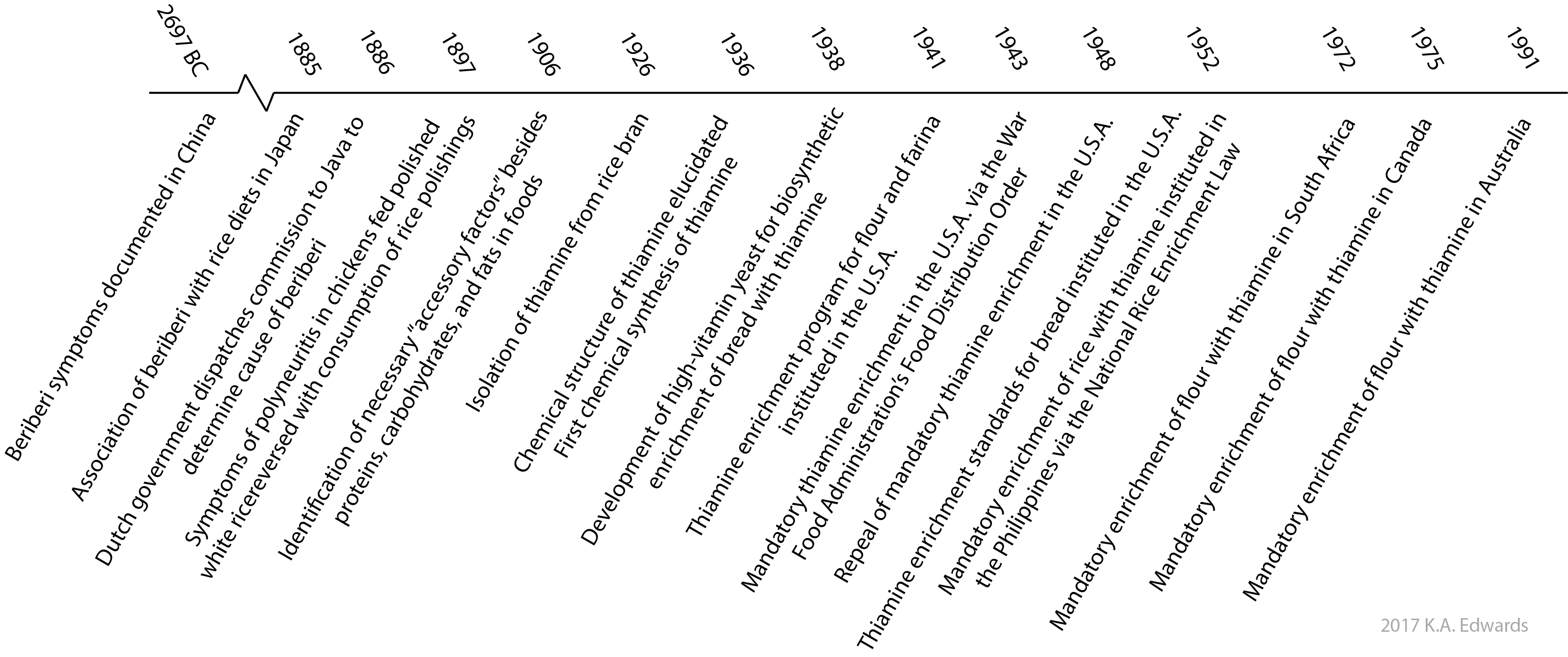
Mammals cannot synthesize thiamine and thus must obtain it from their diets. By contrast, most bacteria, plants, and fungi are able to synthesize thiamine. In fact, in 1938, it was this biosynthetic ability of yeasts that initially made thiamine supplementation in the commercial bread supply economically viable.8 Shortly thereafter in 1941, a formal enrichment program for thiamine, iron, and niacin was instituted in the United States for white flour and farina (milled wheat). In order for a product to be labeled as 'enriched', it needed to contain amounts of these components as specified by the FDA. This action was done to improve the health and productivity of the working population as well as those entering military service. Standards for bread labeled as 'enriched' were introduced in 1952.9 Aside from a brief period in between 1943 and 1946, the sale of enriched products was voluntary on a national level. Certain states, however, made enrichment mandatory. Although many food manufacturers realize the benefits from marketing enriched products and thus manufacture them, enrichment remains voluntary in the United States. In many countries however, enrichment is mandatory. Even with enrichment standards, in some reported cases, there remains considerable fluctuation in the nutritional content of enriched products which may negatively impact consumers.10
Website goal
The aim of this website is to provide a central location for background and recent findings on thiamine deficiency and will be continually updated. Please contact Katie Edwards ![]() if you have suggestions.
if you have suggestions.
References
1. https://ods.od.nih.gov/factsheets/Thiamin-HealthProfessional/
2. http://www.who.int/medicines/publications/essentialmedicines
3. Marks J. Thiamine. A Guide to The Vitamins: Their role in health and disease. Dordrecht: Springer Netherlands; 1975:73-81.
4. McDowell L. Thiamin. In: Vitamins in Animal and Human Nutrition. 2nd ed. Ames: Iowa State University Press; 2000:265-310. Available at: http://www.ucv.ve/fileadmin/user_upload/facultad_agronomia/Producion_Animal/Vitamins_in_Animal_and_Human_Nutrition.pdf
5. Bay AR. Beriberi, Military Medicine, and Medical Authority in Prewar Japan. Japan Review. 2008(20):111-156.
6. Eggersdorfer, M. et al. One hundred years of vitamins - A success story of the Natural Sciences. Angewandte Chemie. 2012;51(52):12960-12990
7. https://www.nobelprize.org/educational/medicine/vitamin_b1/eijkman.html
8. Bradley WB. Thiamine enrichment in the United States. Annals of the New York Academy of Sciences. 1962;98(2):602-606.
9. Dietary Reference Intakes: Guiding Principles for Nutrition Labeling and Fortification. Institute of Medicine (US) Committee on Use of Dietary Reference Intakes in Nutrition Labeling 2003; https://www.ncbi.nlm.nih.gov/books/NBK208880/. Accessed Dec. 31, 2016.
10. LeonGuerrero RT, Gebhardt SE, Holden J, et al. White rice sold in Hawaii, Guam, and Saipan often lacks nutrient enrichment. Journal of the American Dietetic Association. 2009;109(10):1738-1743.