Recent Thiamine Research
Contents
Recent Findings
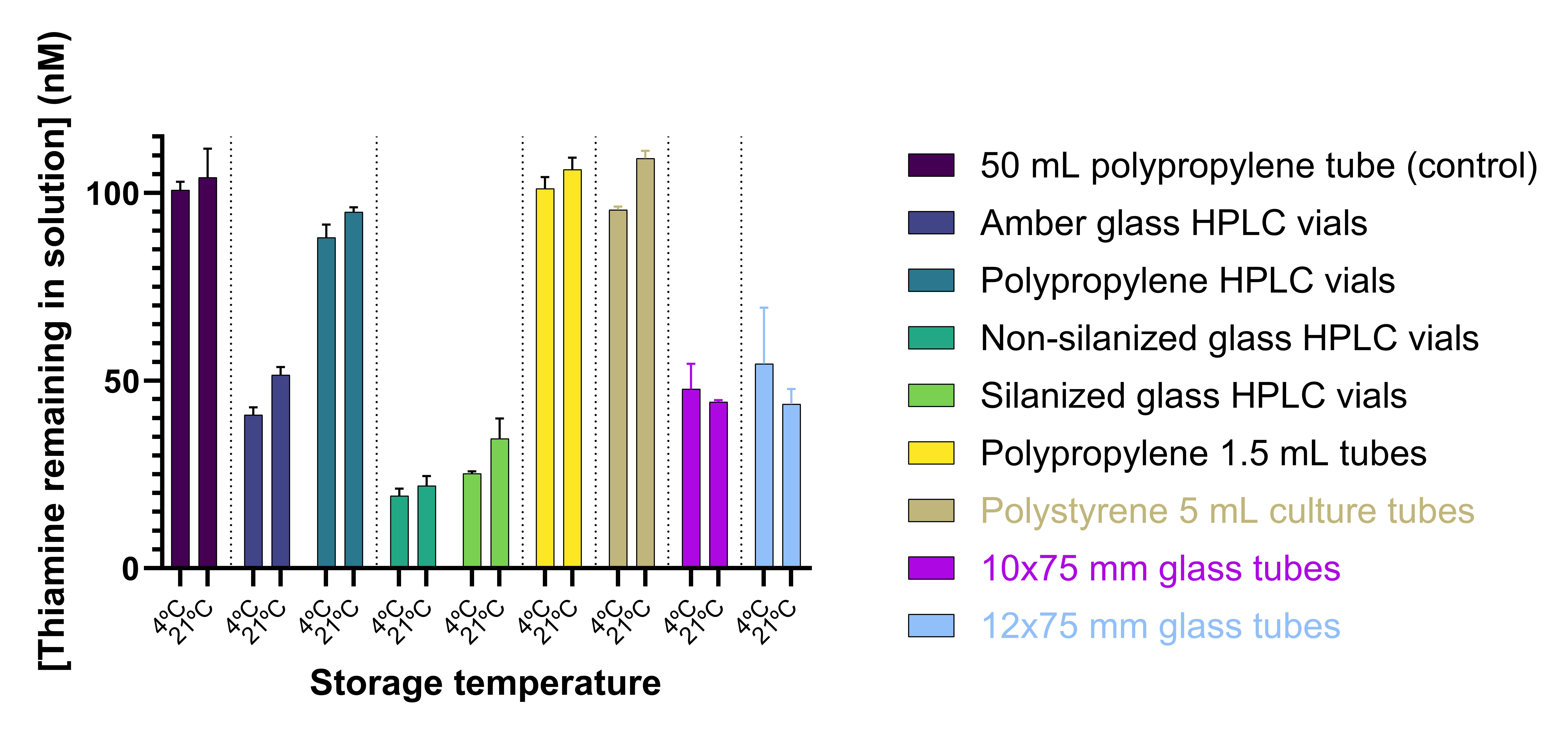
We found significant losses of thiamine to glass containers and filters, reducing the availability of this analyte for downstream analytical assays. Losses to both silanized and non-silanized glass HPLC vials were notable in a short period of time. Similarly, even transient passage of solutions through glass fiber filters resulted in marked losses. This could yield inaccurate standards used in thiamine analysis, inaccurate quantification of thiamine in samples, and possible misinterpretation of thiamine-limited microbial growth experiments.
Certain bacteria produce thiaminase I enzymes, which can break down thiamine into its constitutive thiazole and pyrimidine fragments when in the presence of nucleophilic substances.
Our recent study in Scientific Reports screened various natural products for their ability to enhance and inhibit thiaminase I enzyme activity from Clostridium botulinum overexpressed in Escherichia coli, as well as naturally expressed thiaminase I activity in Paenebacillus thiaminolyticus, Paenebacillus apiarius, and Burkholderia thailandensis.
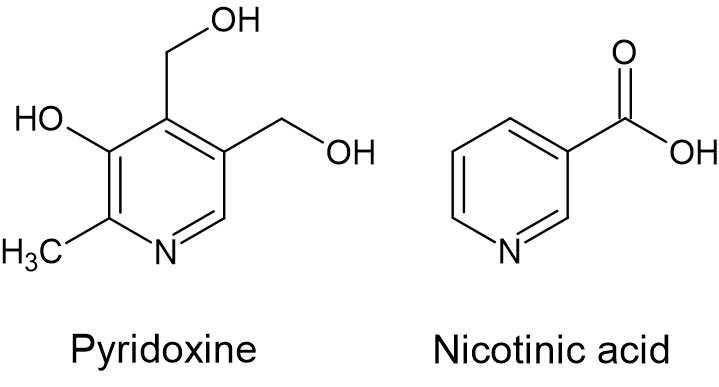
We found that pyridoxine (vitamin B6) was a potent cosubstrate for thiaminase I activity, and to a much lesser degree, nicotinic acid (vitamin B3).
We surprisingly found that despite the presence of these cosubstrates, that thiaminase activity was inhibited in a multivitamin formulation.
We screened various natural inorganic and organic products and identified ascorbic acid and Cu2+ as being potent inhibitors of thiaminase activity, suggesting that thiamine degradation is not a function of only the enzyme presence, but also strongly subject to the influence of dietary composition. Cu2+ irreversibly inhibit thiaminase activity wherease inhibition by ascorbic acid was reversible.
Silver Carp (Hypophthalmichthys molitrix) are one of four species of invasive carp in the Mississippi River Basin and other bodies of water. These fish were imported to the United States from Asia in the 1970's to help control algae in wastewater treatment facilities, but ultimately established themselves and proliferated in natural waters. These fish feed primarily on phytoplankton, consuming ~40% of their body weight per day, which is not insignificant when they commonly weigh between 20 pounds and reach upwards of 90 pounds and three feet in length. Their phytoplankton consumption outcompetes other juvenile and adult fish that feed on the same, impacting these populations as well as predator fish that feed on them. Significant efforts are being made to harvest these fish as animal and human food to reduce their environmental impact and for economic benefit. Our recent study in Current Research in Food Chemistry investigated the levels of thiaminase activity in Silver carp and impact of common food processing steps (e.g.-dehydration, microwaving, baking, freeze-drying) on activity.
As thiamine is needed for the growth of certain microorganisms, the inducement of thiamine deficiency may be a viable supportive treatment for opportunistic fungal infections.12 Yeasts such as Candida albicans and Malassezia pachydermatis are part of the normal microflora in the clinical and veterinary realm, but their overgrowth can cause a variety of health concerns. A 2016 study investigated whether treatment with thiamine analogues such as oxythiamine (Figure 4) could decrease growth of these microorganisms as a possible supportive treatment for more toxic anti-fungal therapies.
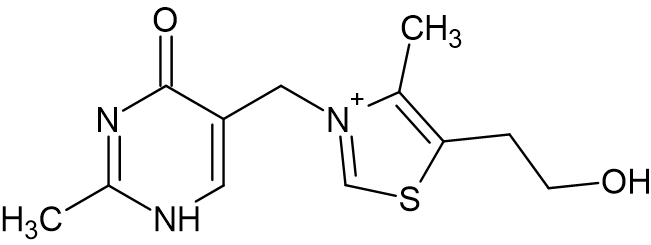
Oxythiamine can be phosphorylated and thus bind within the cofactor site of thiamine diphosphate-dependent enzymes. However, as it cannot generate the ylide form, it lacks biological activity. In M. pachydermatis and S. cerevisiae, the in vitro administration of oxythiamine yielded a reduction in their growth.
As oxythiamine can induce symptoms of thiamine deficiency in the host, it is thus not well-suited for systemic therapy, but may be a viable topical treatment for fungal infections.
Interestingly, although oxythiamine reduced the growth of C. albicans, this organism was least affected by oxythiamine, which is postulated by its greater ability to make use of alternate metabolic pathways.
Borrelia burgdorferi, the causative agent of Lyme disease, reportedly does not require thiamine. B. burgdoferi does not have genes required for the biosynthesis of thiamine, nor transport, nor enzymes that require TDP as a cofactor. Zhang et al found that, while bacteria such as Escherichia coli contained between 3.6-4.8x104 molecules of thiamine per cell, B. burgdoferi contained no detectable thiamine.7
B. burgdoferi, along with other pathogenic microorganisms, infect ticks which serve as vectors for diseases including Lyme disease, Rocky Mountain Spotted Fever, and rickettsiosis in higher organisms. One species of tick infected by B. burgdoferi is the black-legged tick (Ixodes scapularis). Thiamine levels in this tick species are reportedly very low, with only 1.15 pmol thiamine/tick, 2.02 pmol TMP/tick, and no detectable TDP.
These low levels of thiamine-related molecules in the host, in conjunction with the lack of biosynthetic or transport pathways for thiamine in the bacteria, substantiate the viability of the bacteria in the absence of thiamine. B. burgdoferi seems to have bypassed the thiamine requirement of all other organisms by developing alternative pathways to acetyl-CoA and ATP synthesis. Zhang et al postulate that this alternate pathway may have evolved to allow this bacteria to survive in a low-thiamine content host.
Ecological impact of thiamine deficiency Collaborators
Esther Angert, Cornell University, Microbiology
Katie Edwards, Binghamton University, School of Pharmacy and Pharmaceutical Sciences, Cornell University, Microbiology
Clifford Kraft, Cornell University, Department of Natural Resources and the Environment
Eileen Randall, Cornell University, Department of Natural Resources and the Environment