Thiamine Properties
Thiamine Structure
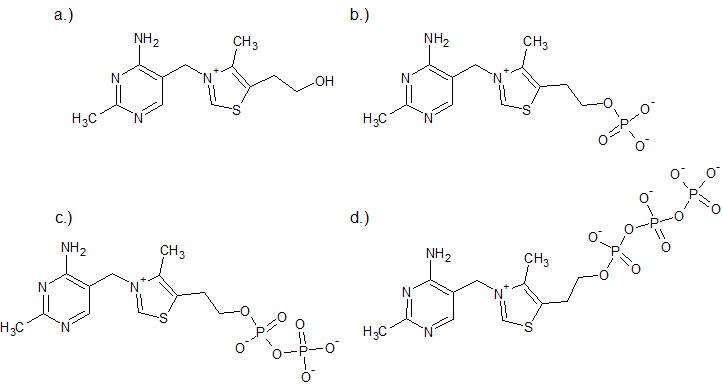
Thiamine consists of 2-methyl-4-aminopyrimidine attached via a methylene group to a thiazole ring, substituted with a methyl group in the 4 position and hydroxyethyl group in the 5 position. Phosphorylated derivatives of the hydroxyl group include thiamine mono-, di-, and triphosphates (TMP, TDP, and TTP, respectively) (Figure 1).
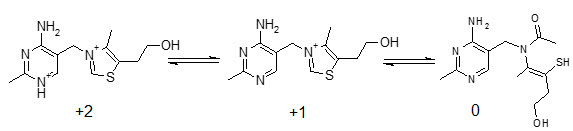
Thiamine is a water soluble vitamin with limited solubility in alcohols and even less solubility in less polar organic solvents. Its charge is pH dependent, but it exists as a cation at physiological pH (Figure 2).
Thiamine Reactivity
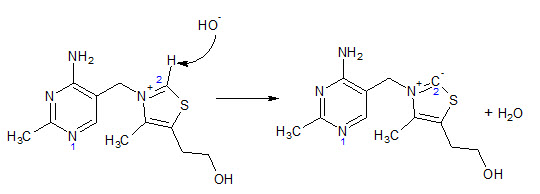
Physiologically, the most important part of thiamine is its thiazole ring. The C2 carbon (that between the N and S of the ring) is slightly acidic meaning that it can donate a proton and become negatively charged (Figure 3). Since this negative charge on the C2 carbon is adjacent to a positive charge on the thiazole nitrogen, this form is termed a ylide. The negative charge formed in this structure allows this carbon to act as a nucleophile in subsequent reactions.
Thiamine diphosphate (otherwise known as thiamine pyrophosphate, TDP, or TPP) is the form of thiamine that is most biologically relevant, serving as a cofactor for several enzymes involved in carbohydrate metabolism. As discussed further on the biochemistry page, these enzymes are involved in reactions which convert carboxylic acids to aldehydes and thioesters as well as break and form C-C bonds. To properly situate in the enzyme cofactor site, the negative charges on the phosphate groups of TDP are coordinated by a divalent metal, most effectively Mg2+ or Ca2+. Further, the cofactor site of the enzymes places TDP in a V-conformation, which situates the amine group of the aminopyrimidine ring in close proximity to the C2 carbon on the thiazole ring. Coupled with a conserved glutamic acid residue in the cofactor site of the enzyme which can form a hydrogen bond with the N1 nitrogen of the pyrimidine ring, the ylide on the thiazole ring that can be formed chemically is instead formed enzymatically.
Publications of interest
Pletcher J, Sax M, Turano A, Chang C-H. Effects of structural variations in thiamin, its derivatives and analogues. Annals of the New York Academy of Sciences. 1982;378(1):454-458.
Soriano EV, Rajashankar KR, Hanes JW, Bale S, Begley TP, Ealick SE. Structural Similarities between Thiamin-Binding Protein and Thiaminase-I Suggest a Common Ancestor. Biochemistry. 2008;47(5):1346-1357.